About
Sagimet is a clinical-stage biopharmaceutical company developing novel fatty acid synthase (FASN) inhibitors that are designed to target dysfunctional metabolic and fibrotic pathways in diseases resulting from the overproduction of the fatty acid, palmitate. FASN is a regulator of lipid synthesis and a key pathway implicated in multiple diseases, such as metabolic dysfunction-associated steatohepatitis (MASH), formerly known as nonalcoholic steatohepatitis (NASH), acne and select forms of cancer.
Our lead drug candidate, denifanstat, is an oral, once-daily pill and selective FASN inhibitor in development for the treatment of MASH, for which there are few approved treatments. We announced positive topline results for FASCINATE-2, a Phase 2b clinical trial of denifanstat in MASH with liver biopsy-based primary endpoints, in January 2024. In October 2024, the FDA granted Breakthrough Therapy designation to denifanstat for the treatment of noncirrhotic MASH with moderate to advanced liver fibrosis (consistent with stages F2 to F3 fibrosis).
FASCINATE-2 was a Phase 2b clinical trial of denifanstat in biopsy-confirmed MASH patients with moderate to advanced fibrosis (F2/F3) at week 52. In this trial, denifanstat, an oral, selective FASN inhibitor, showed statistically significant improvements relative to placebo on both of the primary endpoints of MASH resolution without worsening of fibrosis with ≥2-point reduction in NAS, and ≥2-point reduction in NAS without worsening of fibrosis.
Denifanstat-treated patients also showed statistically significant fibrosis improvement by ≥ 1 stage with no worsening of MASH, and a greater proportion of MRI-derived proton density fat fraction (MRI-PDFF) ≥30% responders relative to placebo.
Denifanstat impacts key drivers of MASH
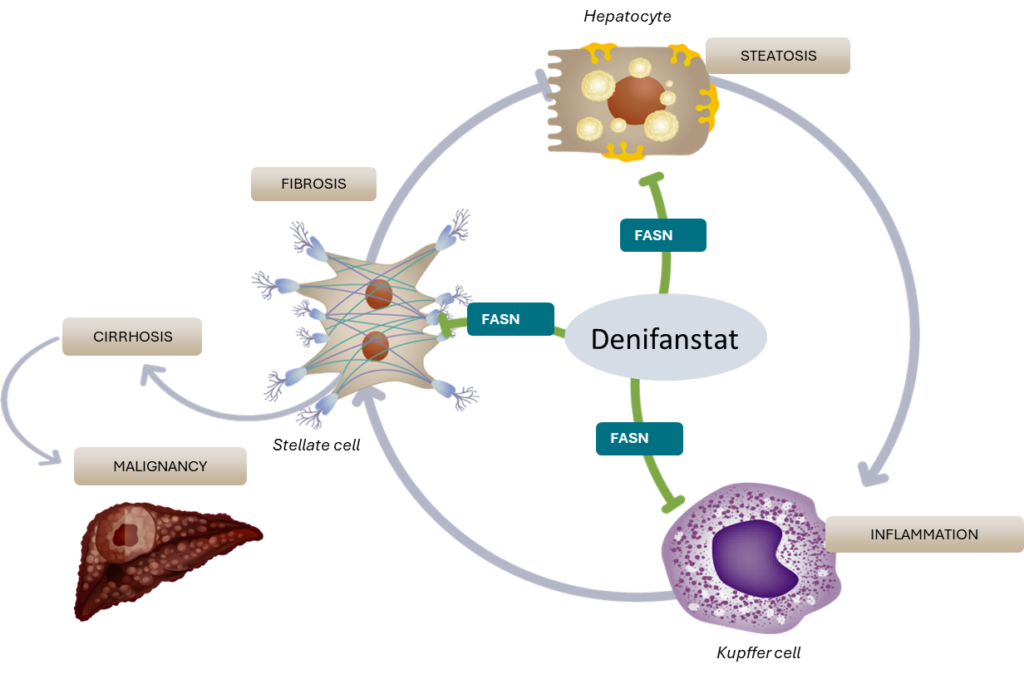
In addition to MASH, we are exploring the use of our FASN inhibitors, which include denifanstat and our pipeline product candidate, TVB-3567, in acne and in select forms of cancer, disease areas in which dysregulation of fatty acid metabolism also plays a key role.
Acne
In June 2025, we reported that denifanstat met all primary and secondary endpoints in a Phase 3 clinical trial for the treatment of moderate to severe acne vulgaris conducted by Sagimet’s license partner Ascletis Bioscience Co. Ltd. (Ascletis) in China. Ascletis reported that denifanstat was generally well-tolerated. The Phase 3 clinical trial (NCT06192264) was a randomized, double-blind, placebo-controlled, multicenter clinical trial in China to evaluate the safety and efficacy of denifanstat for the treatment of patients with moderate to severe acne. The 480 enrolled patients were randomized 1:1 into two treatment arms to receive denifanstat 50mg or placebo, once daily for 12 weeks.
In March 2025, we announced the clearance of our Investigational New Drug (IND) application for a first-in-human Phase 1 clinical trial of our second FASN inhibitor, TVB-3567. TVB-3567 is a potent and selective small molecule FASN inhibitor, planned to enter clinical development for the treatment of acne. Following the IND clearance, we initiated a first-in-human Phase 1 clinical trial of TVB-3567 for development of an acne indication in June 2025.
Cancer
Our FASN inhibitor denifanstat has demonstrated activity against select forms of cancer in pre-clinical and clinical studies.
*Sagimet is derived from a combination of Sagitta and metabolism. In Greek mythology, Sagitta is the arrow used to stop the eagle sent by Zeus to perpetually gnaw on Prometheus’ liver as punishment for gifting fire to humans. Our therapeutic focus targets dysfunctional metabolic pathways.

